Abstract
Introduction: Thromboembolism (TE) is a major toxicity of acute lymphoblastic leukemia (ALL) treatment and contributes to substantial morbidity and mortality. Several germline DNA variants have been associated with TE in adults without cancer. A meta-analysis of genome-wide association studies on TE by Germain et al. (Am J Hum Genet 2015) identified single nucleotide polymorphisms (SNPs) in 8 genes that contribute to increased risk of TE in adults (ABO,F2, F5,F11, FGG,PROCR,TSPAN15, and SLC44A2). De Haan et al. (Blood 2012) found that SNPs in 5 of these (F5, F2, F11, FGG, and ABO) predicted TE almost as well as a 31 SNP-based risk score. However, the impact of these SNPs in patients with cancer, particularly in children, remains uncertain.
Materials and methods: The Nordic Society of Pediatric Hematology and Oncology (NOPHO) ALL2008 protocol for children and adults (1-45 years) included a 3-drug induction (vincristine, doxorubicin, glucocorticosteroid) followed by exposure to asparaginase (1,000 IU/m.sq. i.m.) from week 5 until week 33 (details of therapy in Toft, Leukemia 2018). We collected germline DNA and prospectively registered TE events on 1482 children and adults diagnosed with ALL and treated according to the ALL2008 protocol in seven Nordic and Baltic countries (7/2008-7/2016) (Rank, Blood 2018). Inclusion criteria for TE were i) symptomatic venous or arterial TE confirmed by imaging or by autopsy for TE diagnosed post mortem or ii) asymptomatic TE confirmed by imaging due to other non-TE symptoms and treated with systemic anticoagulation. Based on previously published data and a priori power calculations, we selected and genotyped 5 SNPs: F5 rs6025 (risk allele frequency (RAF) 0.05), F11 rs2036914 (RAF 0.52), FGG rs2066865 (RAF 0.22), and ABO SNPs rs8176719 (RAF 0.39) and rs2519093 (RAF 0.24). Three SNPs (F5 and the two ABO SNPs) were found by imputation, which was done on a subset of patients with European ancestry and included in the NOPHO ALL2008 protocol (N = 1229). We constructed genetic risk scores using a combination of the SNPs.
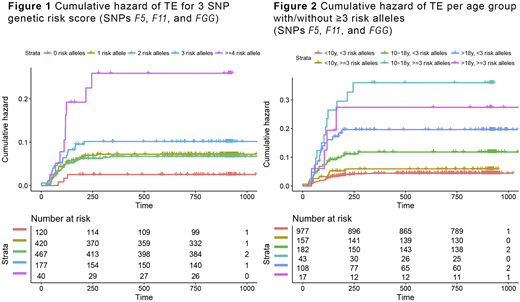
Results: During the ALL treatment period 107 of 1482 patients developed TE (2.5-year cumulative incidence 7.3%, 95% confidence interval (CI) 5.9-8.5). In multivariate Cox regression analysis controlling for age, gender, presence of mediastinal mass and enlarged lymph nodes (N = 1192, whereof TE events 84), we found statistically significant associations with TE development for SNPs F5 rs6025 (hazard ratio (HR) 2.96, 95% CI 1.59-5.48), F11 rs2036914 (HR 1.62, 95% CI 1.18-2.24), and FGG rs2066865 (HR 1.40 95% CI 1.01-1.95), whereas there were no significant associations with ABO SNPs rs8176719 (HR 0.98, 95% CI 0.64-1.51) or rs2519093 (HR 1.06, 95% CI 0.65-1.73). An unweighted 3 SNP risk score based on SNPs F5, F11, and FGG was associated with TE development (HR 1.59, 95% CI 1.27-1.98) (Figure 1). Twenty-six of 217 patients with ≥3 risk alleles developed TE (12.0%, 95% CI 8.1-17.2), compared to 62 of 1007 patients with <3 risk alleles (6.2%, 95% CI 4.8-7.9). The association was strongest in the adolescent age group (10-18 years; HR 1.88, 95% CI 1.35-2.63). Thirteen of 43 adolescents with ≥3 risk alleles developed TE (30.2%, 95% CI 17.8-46.3), compared to 20 of 182 adolescents with <3 risk alleles (11.0%, 95% CI 7.0-16.7) (Figure 2). In adults (>18 years) the proportion who developed TE was quite high in both the group with ≥3 risk alleles (23.5%, 95% CI 7.8-50.2) and with <3 risk alleles (17.6% 95%CI 11.1-26.3). In children (1-10 years) the proportion who developed TE was low in both the group with ≥3 risk alleles (5.7%, 95% CI 2.8-10.9) and with <3 risk alleles (3.2%, 95% CI 2.1-4.8). A weighted 3 SNP genetic risk score based on estimated odds ratios from literature for SNPs F5, F11 and FGG was also associated with TE development (HR 2.84, 95% CI 1.85-4.36). Again, the association was strongest in adolescents (HR 4.20, 95% CI 2.22-7.94).
Conclusion: Based on the strong association between F5 rs6025, F11 rs2036914, and FGG rs2066865 and TE development, not least for adolescents, future preventive measures for TE should target adolescents with ≥3 risk alleles as well as any adults ≥18 years.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal